Research
Our research aimed at creating molecular complexity and diversity from readily-available and affordable starting materials. It revolves around homogeneous catalysis using different tools from supramolecular chemistry.
Hexafluoroisopropanol
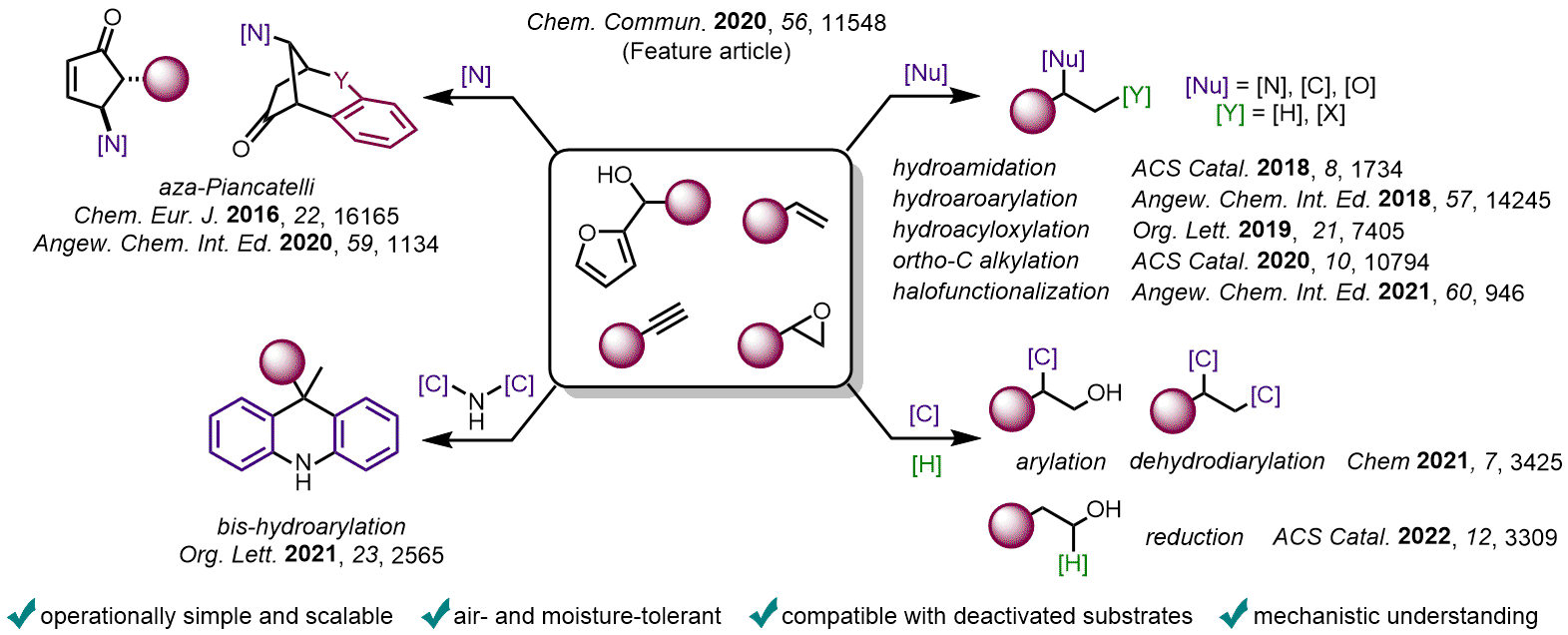
C-C and C-heteroatom bond-forming transformations are essential for constructing the framework of molecules possessing applications in medicinal chemistry, crop science, materials, and cosmetics. Thus, methods that can efficiently assemble these compounds with versatility are imperative. In this context, there is a strong demand from the fine chemicals and pharmaceuticals industry to develop procedures based on transition metal-free reactions using readily available starting materials. The main reasons behind this are the availability and cost of transition-metals and/or electrophiles. In this context, developing new synthetic methods to rapidly build molecular complexity and diversity from simple feedstocks such as alcohols, alkenes and epoxides is truly appealing.
In recent years, I and my group have pushed the boundaries of carbocationic chemistry by using Lewis or Brønsted acids in partnership with hexafluoroisopropanol (HFIP) as a solvent. HFIP has proven to be enabling for many reactions otherwise deemed inaccessible by traditional Brønsted and Lewis acid-catalyzed processes, due to its ability to stabilize cationic species, to strongly donate hydrogen bonds, in addition to its low nucleophilicity and redox stability. It is noteworthy that the role of the Lewis and Brønsted acids in these examples is not to directly activate the nucleophile or the electrophile, but, instead, to enhance the acidity of a H-bond network of HFIP molecules, creating a dynamic supramolecular promoter to activate robust substrates (domino effect).
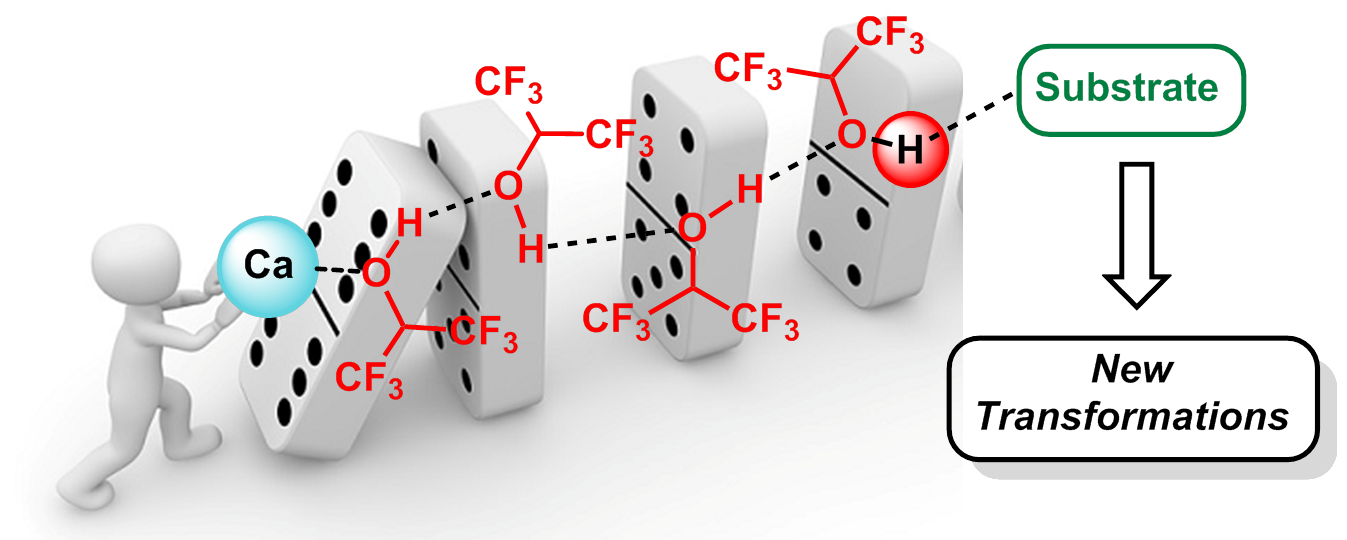
Moreover, due its strong H-bond donor ability, HFIP has the capacity to facilitate the release of Lewis acids trapped by unwanted coordination to the substrate (or the product), allowing the catalytic process to turn over. In the past years, we demonstrated that such type of combination in catalysis was not only an elegant concept but could be used to access original frameworks of interest and, thus, pave the way for potential applications in industry, including medicinal and materials chemistry. Some key examples are presented below. Regarding this topic, DFT computations have also been carried out by my collaborator – Pr. Vincent Gandon – to gain a better understanding of how HFIP operates.
Supramolecular Chemistry
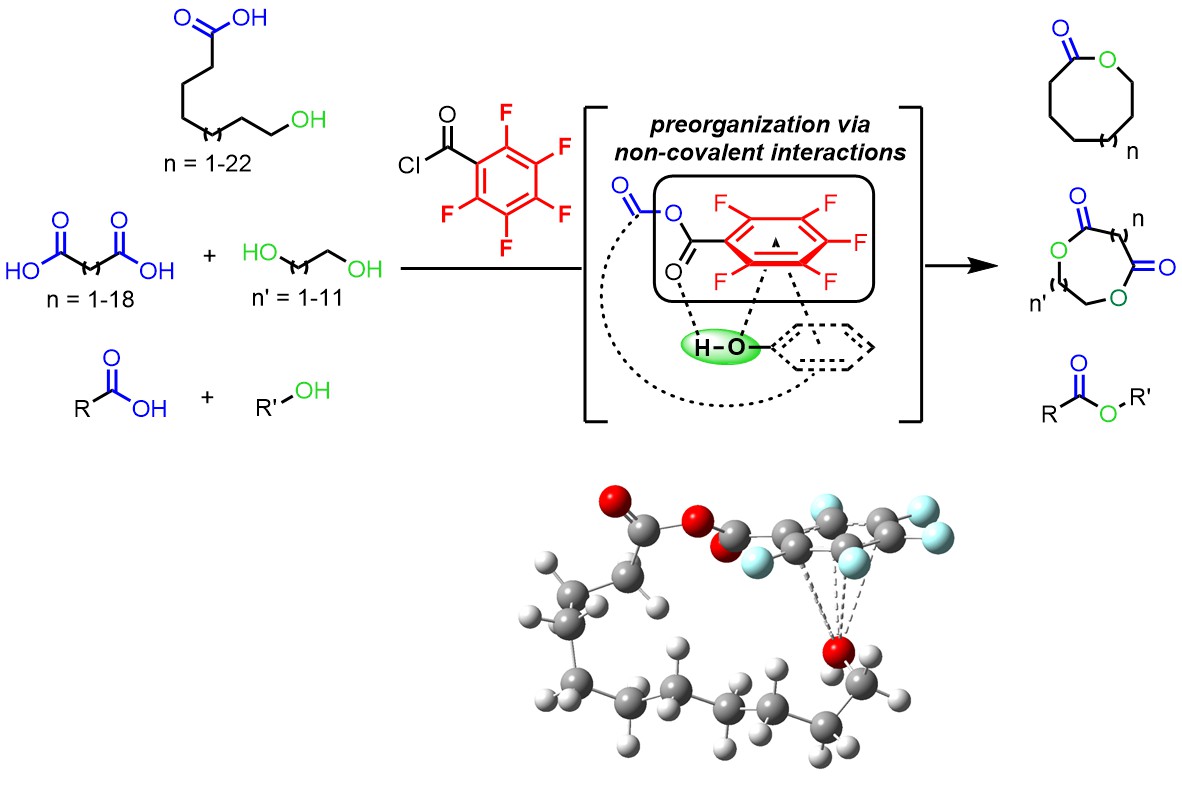
The study of macrolactonization processes has been a steady endeavor for synthetic chemists to access macrocycles that are fundamental in the development of numerous high added-value compounds, notably drugs and fragrances, not mentioning its significant relevance in total synthesis. To achieve this goal, chemists have devised countless methods featuring Brønsted acids, Lewis acids, transition metal complexes or organic molecules as activating agents. However, by taking a close look at the literature, it is noteworthy that most of these methods are in fact complementary in terms of reactivity, often focusing on a specific class of substrates. Thus, a general and cheap promoter system to achieve macrocyclizations towards the formation of both macrolactones and macrodiolides, while being compatible with a broad range of alcohols, notably poorly nucleophilic phenols, was still lacking.
We thus developed a new strategy for the macrolactonization of seco-acids and diacids that efficiently generates a vast library of macrolactone, macrodiolide and ester derivatives, relying on the use of pentafluorobenzoyl chloride as an activating agent. This approach is a step-change advance over the classic macrocyclization strategies reported by Yamaguchi and Shiina, both in terms of practicality and in terms of concept. The key is that the use of a pentafluorophenyl group which allows the pre-organization of the substrate – via lone pair-pi and pi-pi interactions – while increasing the reactivity of the mixed anhydride intermediate, enabling for the first time not only the macrolactonization of seco-acids and the macrodilactonization of diacids with diols but also esterification reactions. In the current economic context, this method does not require the use of any transition metals but an inexpensive activating agent and is a user-friendly tool that allows to remove all the by-products by simple aqueous treatments for a more sustainable chemistry. In addition, to rationalize the superior reactivity of the pentafluorobenzoyl group, we performed extensive mechanistic studies, including kinetic studies, low-temperature NMR and DFT computations in collaboration with Dr. Ilaria Ciofini.
We worked also on the design and application of novel supramolecular organometallic complexes relying on overlooked non-covalent interactions to provide an alternative to current catalytic systems. The project involve the synthesis and characterization of these new complexes and their use in classical and novel (enantioselective) transformations (hydrogenation, hydrosilylation, electrocyclization, etc.).
Photooxygenation of Furans
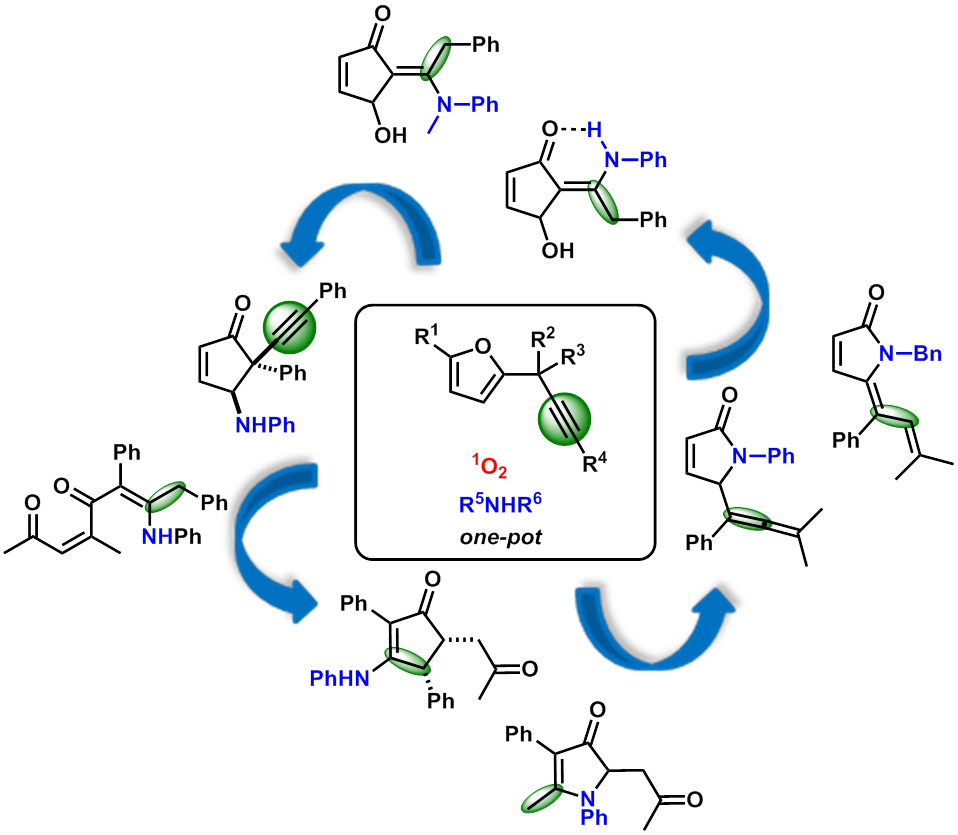
Furans are amongst the most versatile building blocks in organic synthesis. And, one of the major reasons is the ease with which the C-O bonds can be cleaved, creating an avenue for a large variety of poly(hetero)cyclic scaffolds depending on the substrate pattern of the precursors and the reactions conditions. Moreover, the interest for furans lies in the fact that their common precursors, furfural and 5-hydroxymethylfurfural, can be directly obtained from biomass derived carbohydrates such as cellulose and hemicellulose. In that respect, the photooxygenation of furans is particularly attractive as this transformation relies on the simple use of singlet oxygen 1O2, which can be generated from ground state triplet oxygen by visible light in the presence of trace amounts of a suitable photosensitizer (rose bengal, methylene blue, tetraphenylporphyrin, etc.). As part of our interest in the reactivity of furan derivatives, we investigated the reactivity 2-propargylfurans, which had never been explored with respect to photooxygenation. and demonstrated that they could enable access to a large range of nitrogen-containing cyclopentenones and related compounds through a subtle design of substrates.